The SfRBM Foundation was designed to foster and support SfRBM’s education, training, and research programs as well as fund new developments and opportunities in these areas.
In fact, since 1995, SfRBM has provided over $450,000 in Research Mini-Fellowships, Travel, and Young Investigator Awards to support scientists in early stages of their careers.

There are a number of ways to contribute, including one-time donations and Planned Giving. As 2019 comes to a close, please consider including the SfRBM Foundation in your giving plans.
Here are 5 reasons to donate to the SfRBM Foundation Today:
Please call us today at (317) 205-9482 to learn more about Planned Giving Opportunities. 
— Published
Categories: Free Radical Biology and Medicine, Education, SfRBM Trainee Council, Research, Redox Biology
By: Phyllis A. Dennery, MD
How do physicians differ from basic scientists in their approach to diseases? The former provide insights into disease processes and their manifestations whereas, the latter delve deeply into mechanisms and look for potential therapeutic approaches. If we capitalize on these differences, this could lead to an ideal partnership between those who witness disease at the bedside and those who can adeptly use ever-evolving tools to elucidate the mechanistic underpinnings of human pathology.
 Why is this partnership not always successful? Communication is often challenging due to limited understanding of each participants perspective. In fact, the gap is widening between the language spoken by biomedical scientists and clinicians/physician-scientists. With exponential advances in molecular biology and genetics, it has become difficult to incorporate these novel understandings into everyday medical practice. Philosophically, the two groups have different goals. Science for the sake of science is laudable and necessary to organically discover new insights. However, the pressures of finding a solution for diseases exist and are much more vivid for the physician-scientists who want solutions for their patients. Clinicians want to save lives and have immediate impact but are hampered by the fear of injury and/or bad outcomes. Scientists are encouraged to challenge paradigms and to develop new approaches which leads to a new found knowledge without the fear of such consequences. There needs to be a way to bridge the divide between the culture of basic scientists and that of physician-scientists.
Why is this partnership not always successful? Communication is often challenging due to limited understanding of each participants perspective. In fact, the gap is widening between the language spoken by biomedical scientists and clinicians/physician-scientists. With exponential advances in molecular biology and genetics, it has become difficult to incorporate these novel understandings into everyday medical practice. Philosophically, the two groups have different goals. Science for the sake of science is laudable and necessary to organically discover new insights. However, the pressures of finding a solution for diseases exist and are much more vivid for the physician-scientists who want solutions for their patients. Clinicians want to save lives and have immediate impact but are hampered by the fear of injury and/or bad outcomes. Scientists are encouraged to challenge paradigms and to develop new approaches which leads to a new found knowledge without the fear of such consequences. There needs to be a way to bridge the divide between the culture of basic scientists and that of physician-scientists.
Of course, the integration of newfound knowledge of basic science insights into clinical decision making is important to improve patient care. However, once a discovery is made, dissemination and adoption in clinical practice is another significant hurdle which requires community engagement and public health support. This path is riddled with pitfalls. Therefore, it is often difficult to see how certain basic concepts lead to therapeutic interventions and to clinical successes. This has been a big challenge for redox biology in that despite years of clear ascertainment that antioxidants are beneficial to prevent cytotoxicity in various model systems, the applications to large scale human trials have been extremely limited and discouraging. This is likely due to the fact that the human organism is much more complex than any other laboratory-based model and there are many more factors that come into play to alter outcomes, in particular, disparities in healthcare delivery and lack of cultural competence. Nevertheless, a partnership between physicians and scientists may overcome these hurdles by identifying ways to enhance a particular benefit without causing harm.
Recently, we have seen some encouraging success stories around treatment of disease using redox biology concepts. Hopefully, this is the beginning of a new era. The process from discovery to implementations is long and tortuous. A recent publication reviewed the literature and identified papers where a scientific discovery was made followed by its adaptation into healthcare. On the average, it took 17 years for this to happen (1). This means that we have to tolerate the uncertainty between basic discoveries and eventual adaptation into clinical practice for many years and not lose faith. This is not for the faint of heart and requires constant vigilance to continue to focus on the ultimate goal through partnership and collaboration.
Many will argue that we have to conduct science for the sake of science, rather than focus on finding an immediate solution to a disease process. I would say that we have to adopt both approaches. Families, patients, their families and communities rely on us to help improve their health. Without a goal towards therapeutic discoveries, we will extinguish their hopes and erode their faith in us.
How can we then work together as physician-scientists and basic scientists? We can be patient with each other in terms of understanding each other’s language. Oftentimes the physician rolls her eyes when PhD colleagues try to describe a disease process, smugly dismissing the limited understanding. In return, the basic scientist is exasperated by the approaches taken by the physician-scientist to solve a problem, and chastise him for his lack of rigor or his choice of vague assays. Working together and encouraging each other will go farther. Trying to formalize relationships between the most basic and most clinical investigators would make for an synergistic opportunity to accelerate discovery and implementation.
In the past 28 years, it has been my distinct pleasure to be engaged with SfRBM, known previously as the Society for Free Radical Biology and Medicine and The Oxygen Society. I have enjoyed every iteration of this Society and have felt compelled to be an active participant despite feeling inadequate in my lack of formal training in chemistry, biochemistry, molecular biology and genetics. I feel, nonetheless, that we physician-scientists can bring value to the Society in many ways.
As I approach the Presidency of the SfRBM as its first MD leader, I will look forward to working with you and learning from you to speak a common language that enhances scientific discovery and ultimately promotes health.
1. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510-20.
— Published
Categories: Free Radical Biology and Medicine, Redox Biology
By: Eugenio Barone and Marzia Perluigi
Read the full articles in Free Radical Biology and Medicine here: Barone E et al. Free Radic Biol Med, 111:262-269, 2017, Barone E et al. Free Radic Biol Med, 114:84-93, 2018
Accumulation of oxidative damage is a common feature of neurodegeneration that, together with mitochondrial dysfunction, point to the fact that reactive oxygen species are major contributors to loss of neuronal homeostasis and cell death. Among several targets of oxidative stress, free radical-mediated damage to proteins is particularly important in aging and age-related neurodegenerative diseases. In the majority of cases, oxidative stress mediated post-translational modifications cause non-reversible modifications of protein structure that consistently lead to impaired function.
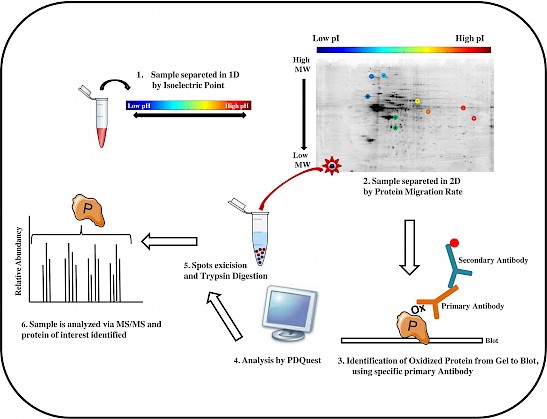
Figure: The workflow of a redox proteomics analysis is showed in six principal steps.
Redox proteomics methods are powerful tools to unravel the complexity of neurodegeneration, by identifying brain proteins with oxidative post-translational modifications that are detrimental for protein function and pathogenesis (Butterfield DA et al. Biochem J, 463:177-89, 2014). Our published studies show evidence of impaired pathways linked to oxidative stress possibly involved in the neurodegenerative process leading to the development of Alzheimer-like dementia (Di Domenico F et al. Antioxid Redox Signal, 26:364-387, 2017). Recently, we also focused on dysregulated pathways underlying neurodegeneration in aging adults with Down syndrome (DS) (Barone E et al. Free Radic Biol Med, 111:262-269, 2017). Interestingly, DS individuals by the age of 40ys are at increased risk to develop Alzheimer disease (AD) like dementia.
Results obtained by the analysis of human specimens and studies from mouse and cellular models of the disease evince a molecular link between protein oxidation/aggregation, the integrity of protein quality control system [proteasome, unfolded protein response (UPR) and autophagy], dysfunction of energy metabolism and neurodegeneration (Di Domenico F et al. Antioxid Redox Signal, 26:364-387, 2017). Many common pathological hallmarks exist between DS and AD including deposition of b-amyloid plaques, Tau-based neurofibrillary tangles, increased oxidative damage, and impaired mitochondrial function, among others. Intriguingly, we propose that all these processes seem to be joined by a “leitmotif” – oxidative stress - since they are all the cause and/or the consequence of increased free radical burden. If low amounts of reactive oxygen species (ROS) can activate the protective cellular apparatus such as the antioxidant and heat shock responses, cell cycle regulation, DNA repair, UPR and autophagy, then chronic exposure to ROS causes irreversible damage to all intracellular macromolecules (Barone E et al. Free Radic Biol Med, 111:262-269, 2017). Among these, protein oxidation impairs multiple cellular functions by a largely irreversible process that results in altered, mostly reduced, protein activity. It is likely that stressed neurons have the challenge of increasing loads of oxidatively damaged proteins, which overwhelm the ability of the proteostasis network. This, in turn, promotes further accumulation of damaged proteins, increasingly prone to aggregation, ultimately resulting in neuronal death. Alteration of protein homeostasis coupled with increasing demand for protein degradation, and reduced ATP production may produce a vicious cycle that may accelerate the neurodegenerative process (Barone E et al. Free Radic Biol Med, 111:262-269, 2017, Barone E et al. Free Radic Biol Med, 114:84-93, 2018).Therapeutic strategies aimed at preventing/reducing multiple components of processes leading to accumulation of oxidative damage will be critical in future studies.
Laboratory of Redox Biochemistry in Neuroscience, Department of Biochemistry, Sapienza University of Rome, Email: Eugenio.barone@uniroma1.it
— Published
Category: Redox Biology
By Dr. Marcus S. Cooke, Ph.D., FRCPath of Florida International University
Luke Skywalker: “I won't fail you! I'm not afraid.”
Yoda: “Oh! You will be. You will be.”
Similar to Luke’s training by Yoda, the PhD is like an apprenticeship, students are mentored and supported throughout, but this is gradually withdrawn, as they become more accomplished, culminating with being an expert in the field, and an independent researcher. On this basis, selection of the right supervisor for you is as vital as selecting the right project area. The successful pursuit of a doctoral degree is a two-way street that requires good alignment between your objectives and those of your supervisor, as well as your progressive capacity to deal with failure and rejection (it’s a fact of life for researchers, I’m afraid). 
A PhD requires fulltime dedication to the project. Before considering undertaking a PhD a prospective student must be willing to devote themselves to the project, understanding that experiments sometimes require working outside business hours. You may possess a great deal of practical experience, but this experience by itself is not sufficient to earn the degree. The project fails or succeeds based on the effort, and ideas from the student, guided and supported by the supervisor. In order to have the required level of commitment, the chosen project on which you work must really excite you – this will provide the motivation to keep going, particularly when experiments prove challenging (i.e. techniques stop working for no apparent no reason!). There will be lows, but there will also be highs, for example when your abstract gets accepted for an international meeting, or your first manuscript gets accepted (it feels good whether it’s your first or 81st). This strengthening of your character will surely enrich your career and life.
The kinds of wet lab projects offered in the broad area of Biomedical Sciences do not readily lend themselves to a part-time degree, but that is not to say it is not possible. A PhD in the Biomedical Sciences means 3-4+ years of intensive research in the lab – sounds like plenty of time, right? But the time flies by in which you must have generated a significant amount of novel data, addressing an important research question. Also, don’t think that a PhD is simply an extension of a Master’s degree, only longer. It is completely different, with objectives, timelines and expectations of progress toward scientific independence that are not seen at the Master’s level. 
Good organization of personal time will be needed, such that you can improve skills that are just as important as your experimental work. This includes: keeping up with the literature about the subject, improving scientific writing, cultivating attention to detail, attending seminars and workshops in and outside your institution, and very importantly, mastering your own oral communication skills. Most institutions demand a minimum of two original data manuscripts, and a review article to submit towards your PhD. These will need to survive the scrutiny of peer review, which will train your capacity to receive criticism and to work proactively on improving the quality of your research.
A huge amount of self-motivation is required to withstand the intrinsic challenges of developing a scientific project toward thinking independently. The structured, taught element of the PhD is minor, in comparison to the time spent in the lab, and just provides a general grounding to get improve your knowledge base. The real learning and training comes in the lab where research skills are learnt firsthand, and tested on a regular basis through the experiments required to test your hypothesis.
Still wanting to do a PhD in the Biomedical Sciences? Get in touch with prospective mentors, discuss with them theirs and your research and career expectations, and go for it! If you are intrinsically thrilled to investigate Nature, you will not be hampered by the high level of commitment required in this career path. With the right PhD training (irrespective of the discipline), you will be well prepared for a wide variety of career options, though giving you skills which allow you to think, speak, write, and problem-solve in a particular way that makes you an asset to any organization.
— Published
Categories: Research, Free Radical Biology and Medicine, SfRBM Trainee Council, Redox Biology
By: Alicia J. Kowaltowski, M.D., Ph.D., Universidade de São Paulo
Obesity enhances the incidence and prevalence of many diseases, but is still increasing worldwide. Our group is focused on understanding mechanisms involved in the protective effects of maintaining a normal body weight, in order to uncover new targets for obesity-related diseases. We do this by studying rodents that eat ad libitum (as much as they want) and develop obesity versus animals on a calorically-restricted diet. Calorically-restricted rodents have been shown to have less oxidative damage and age-related diseases, as well as extended lifespans. 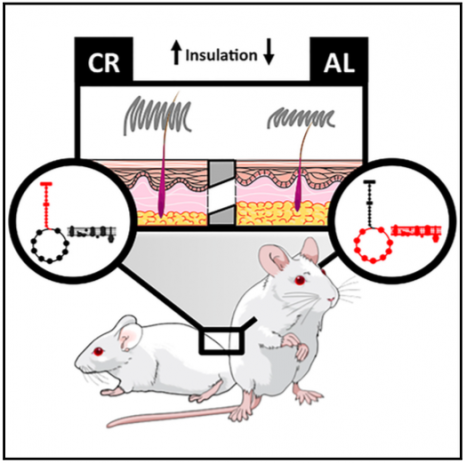
Many of the effects of caloric restriction, predictably, involve changes in energy metabolism and mitochondria, the central hub of energy metabolism. Recently, while investigating mechanisms in which calorie restriction prevents excitotoxic neuronal death (Amigo et al., 2017), we found that mitochondria from calorically-restricted animals can take up Ca2+ ions faster and in larger quantities. The higher buffering capacity these mitochondria have promotes protection against neuronal death caused by excessive Ca2+ in the cytosol.
Because this is a completely new effect of caloric restriction, we decided to investigate if mitochondrial Ca2+ uptake was also modified in the liver (Menezes-Filho et al., 2017). We found that liver mitochondria from calorie restricted mice could also take up more Ca2+, and that this was related to protection against ischemic damage. Overall, the finding that caloric intake affects mitochondrial Ca2+ uptake is interesting because Ca2+ is a well-known modulator of energy metabolism and mitochondrial oxidant production. Based on these initial findings, we hope to uncover more connections between metabolic regulation, redox state, caloric intake and Ca2+ in futures studies.
While studying calorically-restricted mice, we noticed that they had different fur from obese ad libitum fed animals. We then decided to investigate changes in the skin and fur of calorie restricted animals, and found many modifications, both in the structure and metabolism of the skin. One interesting finding was that the fur in calorically-restricted mice has a higher insulation capacity, and is necessary for them to thrive: shaving calorie restricted animals makes them lose muscle mass. We are not sure how these results relate to humans, who aren´t very furry, but believe this is an evolutionary adaptation to deal with heat loss. Although the skin is our largest organ, it is not often studied, and there are almost no related findings. We hope that with this study and the development of methodology to monitor metabolism in the skin, more interest will arise.
Amigo I, Menezes-Filho SL, Luévano-Martínez LA, Chausse B, Kowaltowski AJ. Caloric restriction increases brain mitochondrial calcium retention capacity and protects against excitotoxicity. Aging Cell. 2017 16:73-81.
Forni MF, Peloggia J, Braga TT, Chinchilla JEO, Shinohara J, Navas CA, Camara NOS, Kowaltowski AJ. Caloric restriction promotes structural and metabolic changes in the skin. Cell Rep. 2017 20:2678-2692.
Menezes-Filho SL, Amigo I, Prado FM, Ferreira NC, Koike MK, Pinto IFD, Miyamoto S, Montero EFS, Medeiros MHG, Kowaltowski AJ. Caloric restriction protects livers from ischemia/reperfusion damage by preventing Ca2+-induced mitochondrial permeability transition. Free Radic Biol Med. 2017 110:219-227.
— Published
Category: Redox Biology