To Experienced Mentors,
 My principal investigator (PI) helped me with my writing, the postdocs in lab taught me bench science, and my fellow grad students helped me better communicate my science. I’m going to be in charge of training an undergrad on a project which will be an extension of my dissertation work. I’m excited, and my PI thinks I am ready for this responsibility, but I don’t know if I am going to be good at training as this is my first time as a science mentor. Should I be worried?
My principal investigator (PI) helped me with my writing, the postdocs in lab taught me bench science, and my fellow grad students helped me better communicate my science. I’m going to be in charge of training an undergrad on a project which will be an extension of my dissertation work. I’m excited, and my PI thinks I am ready for this responsibility, but I don’t know if I am going to be good at training as this is my first time as a science mentor. Should I be worried?
Sincerely,
A Nervous New Mentor
Dear A Nervous New Mentor,
First off, congratulations on taking this leadership role in your lab. Training freshmen scientists is one of the most important steps in maintaining the success of not only individual research programs, but scientific progress in general. That being said, training a new lab member as a graduate student or even a postdoc for the first few times can be challenging because rarely will you have someone providing constructive criticism as you employ your own method of training.
Even though this is your first time in a training position, know that your PI trusts you at this point in your career to be able to handle this responsibility. This is not to say there won’t be speed bumps and some additional demands on your already busy lifestyle. But that is where you can utilize your mentors’ help. Never be afraid to ask for advice from your PI and other colleagues who have had the opportunity to train new lab members. Use their advice to mold your own personal training strategy, and over time with trial and error you can improve your mentoring strategy. Even seasoned investigators sometimes have to modify the way they teach based on the available resources and personal characteristics of the trainee.
So to answer your question, don’t be worried. Instead, utilize your skills as a scientist to be observant and know that some hardships and failures may come up that you will have to find solutions to implement.
Sincerely,
An Experienced Mentor (who is still learning a few tricks)
— Published
Categories: Education, Redox Biology
Click here to view the complete article in Redox Biology
Most of the SOD mimics thus far developed belong to the classes of Mn-(MnPs) and Fe porphyrins(FePs), Mn(III) salens, Mn(II) cyclic polyamines and metal salts. Due to their remarkable stability we have predominantly explored Mn porphyrins, aiming initially at mimicking kinetics and thermodynamics of the catalysis of O2− dismutation by SOD enzymes. Several MnPs are of potency similar to SOD enzymes. The in vivo bioavailability and toxicity of MnPs have been addressed also.
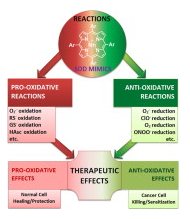 Numerous in vitro and in vivo studies indicate their impressive therapeutic efficacy. Increasing insight into complex cellular redox biology has been accompanied by increasing awareness of complex redox chemistry of MnPs. During O2− dismutation process, the most powerfulMn porphyrin-based SOD mimics reduce and oxidize O2− with close to identical rate constants. MnPs reduce and oxidize other reactive species also (none of them specific to MnPs), acting as reductants (antioxidant) and pro-oxidants.
Numerous in vitro and in vivo studies indicate their impressive therapeutic efficacy. Increasing insight into complex cellular redox biology has been accompanied by increasing awareness of complex redox chemistry of MnPs. During O2− dismutation process, the most powerfulMn porphyrin-based SOD mimics reduce and oxidize O2− with close to identical rate constants. MnPs reduce and oxidize other reactive species also (none of them specific to MnPs), acting as reductants (antioxidant) and pro-oxidants.
Distinction must be made between the type of reactions of MnPs and the favorable therapeutic effects we observe; the latter may be of either anti- or pro-oxidative nature. H2O2/MnP mediated oxidation of protein thiols and its impact on cellular transcription seems to dominate redox biology of MnPs. It has been thus far demonstrated that the ability of MnPs to catalyze O2−dismutation parallels all other reactivities (such as ONOO− reduction) and in turn their therapeutic efficacies.
Assuming that all diseases have in common the perturbation of cellular redox environment, developing SOD mimics still seems to be the appropriate strategy for the design of potent redox-active therapeutics.
— Published
Categories: Education, Redox Biology
By: Dean P. Jones, Emory University
Click here to view the full article in the journal Redox Biology
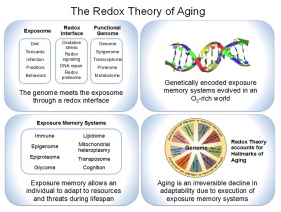 Metazoan genomes encode exposure memory systems to enhance survival and reproductive potential by providing mechanisms for an individual to adjust during lifespan to environmental resources and challenges. These systems are inherently redox networks, arising during evolution of complex systems with O2 as a major determinant of bioenergetics, metabolic and structural organization, defense, and reproduction. The network structure decreases flexibility from conception onward due to differentiation and cumulative responses to environment (exposome). The redox theory of aging is that aging is a decline in plasticity of genome–exposome interaction that occurs as a consequence of execution of differentiation and exposure memory systems. This includes compromised mitochondrial and bioenergetic flexibility, impaired food utilization and metabolic homeostasis, decreased barrier and defense capabilities and loss of reproductive fidelity and fecundity. This theory accounts for hallmarks of aging, including failure to maintain oxidative or xenobiotic defenses, mitochondrial integrity, proteostasis, barrier structures, DNA repair, telomeres, immune function, metabolic regulation and regenerative capacity.
Metazoan genomes encode exposure memory systems to enhance survival and reproductive potential by providing mechanisms for an individual to adjust during lifespan to environmental resources and challenges. These systems are inherently redox networks, arising during evolution of complex systems with O2 as a major determinant of bioenergetics, metabolic and structural organization, defense, and reproduction. The network structure decreases flexibility from conception onward due to differentiation and cumulative responses to environment (exposome). The redox theory of aging is that aging is a decline in plasticity of genome–exposome interaction that occurs as a consequence of execution of differentiation and exposure memory systems. This includes compromised mitochondrial and bioenergetic flexibility, impaired food utilization and metabolic homeostasis, decreased barrier and defense capabilities and loss of reproductive fidelity and fecundity. This theory accounts for hallmarks of aging, including failure to maintain oxidative or xenobiotic defenses, mitochondrial integrity, proteostasis, barrier structures, DNA repair, telomeres, immune function, metabolic regulation and regenerative capacity.
— Published
Categories: Education, Redox Biology
 As scientists we spend an enormous amount of time and effort promoting our findings and their relevance to the rest of the world. We are competitive, and like journalists, we don’t like to get scooped on a story. Whether you’ll admit it to your friend in a neighboring lab, we are regularly vying against one another for limited funding resources, space in top tier journals, speaking engagements, and prestigious awards. So it’s no surprise we, as scientists, are skilled at promoting our work and research. Besides merely promoting your jargon-laden work to peers, it is also useful to actively engage the public to teach facts and dispel myths. Social media provides just such a vehicle to do so, and to build your professional network at the same time.
As scientists we spend an enormous amount of time and effort promoting our findings and their relevance to the rest of the world. We are competitive, and like journalists, we don’t like to get scooped on a story. Whether you’ll admit it to your friend in a neighboring lab, we are regularly vying against one another for limited funding resources, space in top tier journals, speaking engagements, and prestigious awards. So it’s no surprise we, as scientists, are skilled at promoting our work and research. Besides merely promoting your jargon-laden work to peers, it is also useful to actively engage the public to teach facts and dispel myths. Social media provides just such a vehicle to do so, and to build your professional network at the same time.
Many prominent scientists such as Richard Dawkins and Neil deGrasse Tyson have already caught on and promote their “brand” through social media to their millions of friends and followers. They don’t need to publish a peer reviewed paper or book, or attend a conference to instill us with grains of good humored wit; now we can follow them, like disciples, in real time. If academics don’t already get it, then here are the top 5 reasons that scientists should embrace social media:
 Promote your work – Use a tweet to draw attention to a recently accepted paper or an appearance at a conference. This publicity can lead to increased views, downloads and citations of your work. I recently tweeted about a paper that our group published late last year and that simple action yielded at least 7,000 impressions. You shouldn’t be surprised when one of your papers receives more than the expected number of literature citations solely because of this added real time exposure of the work to a relevant audience.
Promote your work – Use a tweet to draw attention to a recently accepted paper or an appearance at a conference. This publicity can lead to increased views, downloads and citations of your work. I recently tweeted about a paper that our group published late last year and that simple action yielded at least 7,000 impressions. You shouldn’t be surprised when one of your papers receives more than the expected number of literature citations solely because of this added real time exposure of the work to a relevant audience.— Published
Categories: Education, Redox Biology
By: Helmut Sies
Published in the journal Redox Biology. Click here to view the full article.
 “Oxidative stress” as a concept in redox biology and medicine has been formulated in 1985; at the beginning of 2015, approx. 138,000 PubMed entries show for this term. This concept has its merits and its pitfalls. Among the merits is the notion, elicited by the combined two terms of (i) aerobic metabolism as a steady-state redox balance and (ii) the associated potential strains in the balance as denoted by the term, stress, evoking biological stress responses. Current research on molecular redox switches governing oxidative stress responses is in full bloom.
“Oxidative stress” as a concept in redox biology and medicine has been formulated in 1985; at the beginning of 2015, approx. 138,000 PubMed entries show for this term. This concept has its merits and its pitfalls. Among the merits is the notion, elicited by the combined two terms of (i) aerobic metabolism as a steady-state redox balance and (ii) the associated potential strains in the balance as denoted by the term, stress, evoking biological stress responses. Current research on molecular redox switches governing oxidative stress responses is in full bloom.
The fundamental importance of linking redox shifts to phosphorylation/dephosphorylation signaling is being more fully appreciated, thanks to major advances in methodology. Among the pitfalls is the fact that the underlying molecular details are to be worked out in each particular case, which is bvious for a global concept, but which is sometimes overlooked. This can lead to indiscriminate use of the term, oxidative stress, without clear relation to redox chemistry. The major role in antioxidant defense is fulfilled by antioxidant enzymes, not by small-molecule antioxidant compounds. The field of oxidative stress research embraces chemistry, biochemistry, cell biology, physiology and pathophysiology, all the way to medicine and health and disease research.
— Published
Category: Redox Biology