By: Ines Batinic-Haberle and Ivan Spasojevic
After 20 years of bench and preclinical work on Mn porphyrins (MnPs), we are thrilled to see that our compounds  have finally reached maturity and entered four clinical trials. We have first designed, synthesized and characterized numerous MnPs, FePs and other metal complexes, which allowed us to establish structure-activity relationship for their SOD-like activities and identify cationic charges in ortho nitrogen positions of peripheral pyridyl groups of MnPs as a key property that enables their high ability to catalyze O2.- dismutation.
have finally reached maturity and entered four clinical trials. We have first designed, synthesized and characterized numerous MnPs, FePs and other metal complexes, which allowed us to establish structure-activity relationship for their SOD-like activities and identify cationic charges in ortho nitrogen positions of peripheral pyridyl groups of MnPs as a key property that enables their high ability to catalyze O2.- dismutation.
Since then, three lead drugs have been most frequently studied: MnTE-2-PyP5+(AEOL10113, BMX-010), MnTnHex-2-PyP5+ and MnTnBuOE-2-PyP5+(BMX-001) (Figure, R, ortho pyridyl substituents are ethyl, n-hexyl and butoxyethyl, respectively).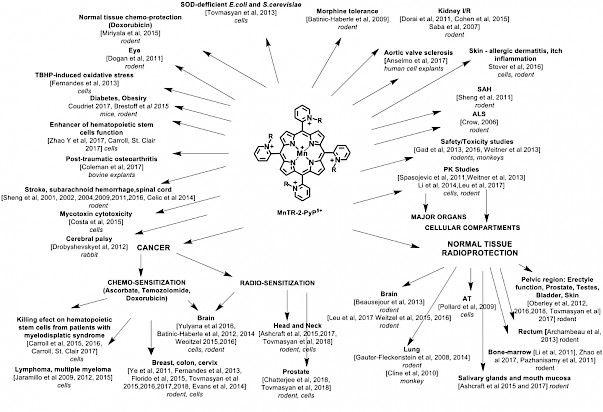 BMX-001 is now tested on suppression of normal tissue injuries in three clinical trials concerning the treatment of various cancers (submitted for publication). Originally designed as SOD-mimics, the biologically compatible reduction potential of Mn in MnP enables broad interactions with other species and redox signaling pathways. In collaboration with Tome’s group, we demonstrated catalysis of S-glutathionylation of Cys residues of NF-кB by MnP/H2O2/GSH, leading to NF-кB inactivation. In addition to NF-кB, S-glutathionylation of Cys was also demonstrated for signaling proteins in our redox proteomics study on 4T1 breast cancer cells treated with the MnP/ascorbate source of H2O2: p38MAPK, protein phosphatase 2A, Keap1, peroxiredoxins 5 and 6, glutaredoxin 3 and 5 and thioredoxin 1, etc.
BMX-001 is now tested on suppression of normal tissue injuries in three clinical trials concerning the treatment of various cancers (submitted for publication). Originally designed as SOD-mimics, the biologically compatible reduction potential of Mn in MnP enables broad interactions with other species and redox signaling pathways. In collaboration with Tome’s group, we demonstrated catalysis of S-glutathionylation of Cys residues of NF-кB by MnP/H2O2/GSH, leading to NF-кB inactivation. In addition to NF-кB, S-glutathionylation of Cys was also demonstrated for signaling proteins in our redox proteomics study on 4T1 breast cancer cells treated with the MnP/ascorbate source of H2O2: p38MAPK, protein phosphatase 2A, Keap1, peroxiredoxins 5 and 6, glutaredoxin 3 and 5 and thioredoxin 1, etc.
Most recently, in a comprehensive mouse breast cancer cellular and mouse study, we explored cationic MnPs (BMX-001, BMX-010), GC4403 (analog of GC4419), Mn(III) salen (EUK-8) and a porphyrin-based but not an SOD mimic, yet frequently studied, anionic MnTBAP3-. Only cationic MnPs, which catalyze ascorbate oxidation and protein S-glutathionylation, radiosensitize breast cancer to radiation, and ascorbate enhances such effect (Tovmasyan et al, Antioxid Redox Signal. 2018 Feb 1. doi: 10.1089/ars.2017.7218). Altogether, our studies show that S-glutathionylation of Cys residues is a major mechanism of action of MnPs. Multiple studies by us and others have shown that the more potent the SOD mimic is, the more reactive it is and the larger are its beneficial therapeutic effects whereby it protects normal tissue from oxidative stress injury while suppresses tumor growth (Figure).
Our pharmacokinetic and efficacy studies showed that differential effects originate from distinct partition of MnP in tissues as well as from tissue-specific redox environments. While therapeutic effects are those commonly associated with antioxidants, the mechanism of action associated with our MnPs is pro-oxidative – we thus tend to call such compounds more correctly redox-active therapeutics than antioxidants.
Departments of Radiation Oncology1 and Medicine2, 3PK/PD Pharmaceutical Shared Resource, Duke Cancer Institute, Duke University School of Medicine, Durham, NC 27707, USA
— Published