Scientists at Sanford Research have just developed a new model that could have lasting implications for some of the world’s most dangerous diseases like cancer, diabetes, neurodegeneration, heart disease, and respiratory distress syndrome.
The new study, led by Peter Vitiello, PhD, Associate Scientist, Children's Health Research Center at Sanford Research, was recently published in Free Radical Biology and Medicine, a journal of the Society for Redox Biology and Medicine (SfRBM).
Many tissue processes and disease pathologies in the human body are influenced by disturbances in cellular redox, an imbalance in the generation and detoxification of chemical oxidants. Thioredoxin-1 (Trx1) is an antioxidant enzyme synthesized by every human cell to detoxify proteins damaged by chemical oxidants. Understanding fundamental molecular functions of Trx1 may provide new therapeutic strategies to prevent disease progression during cellular redox imbalance.
To understand how Trx1 functions during redox alterations and disease pathogenesis, the study described a novel mouse genetically engineered to identify proteins detoxified by Trx1 in lung.
“As we better understand how molecules sense and regulate cellular responses to redox imbalances at the core of so many human disease, we can then improve therapies and treatments that will save lives,” said Dr. Peter Vitiello an Associate Scientist at Sanford Research and SFRBM member. “By creating this transgenic mouse designed to identify proteins targeted by thioredoxin-1, we can learn new information about molecular responses in cells during redox perturbations which we hope will lead to new therapeutic approaches that hinder progression of diseases such as respiratory distress syndrome and neurodegeneration.”
Specifically, Dr. Vitiello’s research team engineered a mutation in the thioredoxin-1 mouse gene that stabilizes interactions with proteins targeted for detoxification by thioredoxin-1. The mutant thioredoxin-1 is then purified along with any bound proteins which are subsequently identified by mass spectrometry. To demonstrate how this technology can be applied to understand disease processes, transgenic mice were treated with excess oxygen to cause lung injury, and Dr. Vitiello found that there were unique sets proteins bound to thioredoxin-1 which were exclusively detected in either control of treatment groups.
— Published
Approximately 2.9 million people in the United States suffer from epilepsy, according to the CDC. For patients living with this diagnosis and their doctors it is often difficult to predict the onset or progression of chronic seizures. Thanks to a newly published study from the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences at the Anschutz Medical Campus, that may be changing.
 The study, led by Drs. Manisha Patel and Li-Ping Liang of the University of Colorado, was recently published in Redox Biology, a journal of the Society for Redox Biology and Medicine (SFRBM).
The study, led by Drs. Manisha Patel and Li-Ping Liang of the University of Colorado, was recently published in Redox Biology, a journal of the Society for Redox Biology and Medicine (SFRBM).
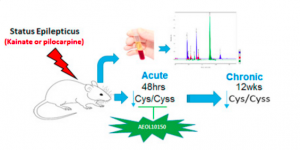
The study was designed to determine if the ratio of reduced and oxidized forms of an amino acid, cysteine and cystine respectively, could serve as an accurate biomarker to predict the onset or progression of seizures associated with epilepsy. Using a rat model, it was determined that decreased cysteine/cystine ratio in plasma may serve as a redox biomarker in epilepsy.
Specifically, seizures were chemically-induced in rats, which were then monitored closely for behavioral changes. Plasma was then taken from the rats 48 hours and 12 weeks after treatment to mimic acute and chronic epileptic conditions. It was found that cysteine/cystine ratio was an accurate redox biomarker for epilepsy. Plasma cysteine/cystine was reduced over 60% in rats with acute epileptic responses and over 37% in rats with chronic epilepsy. Interestingly, cysteine/cystine ratio was unaltered in rats also treated with an antioxidant known to prevent epileptic brain injury.
“Currently the field of epilepsy lacks peripheral blood-based biomarkers that could predict the onset or progression of chronic seizures following an epileptogenic injury,” said Dr. Manisha Patel a Professor at the University of Colorado School of Pharmacy and SFRBM Member. “We are confident that this study is a significant step toward changing this, and will one day help those living with temporal lobe epilepsy.”
About Dr. Patel’s lab
The overarching theme of research in Dr. Patel’s laboratory is to understand the role of redox and metabolic mechanisms in epilepsy. Her laboratory has provided compelling evidence for the role of reactive species and mitochondrial dysfunction in animal models of epilepsy. Furthermore, her research has identified reactive species as novel targets for co-morbidities of epilepsy.
About the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences
Since its inception in 1911, the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences has experienced numerous milestones and is ranked in the top 20 percent of all schools of pharmacy in the country, and fourth in the nation for total NIH funding. Committed to pharmaceutical education, research and patient care, the School educates students in the properties of medicinal agents, the biology of disease, the actions of drugs, and best practices for clinical and therapeutic uses of drugs. Located at the Anschutz Medical Campus, the University of Colorado is the only completely new education, research and patient care facility in the nation today.
— Published
Categories: Free Radical Biology and Medicine, Redox Biology, Research
Click here to read the full study in the journal: Free Radical Biology and Medicine
Cellular redox balance plays a significant role in the regulation of hematopoietic stem-progenitor cell (HSC/MPP) self-renewal and differentiation. Unregulated changes in cellular redox homeostasis are associated with the onset of most hematological disorders. However, accurate measurement of the redox state in stem cells is difficult because of the scarcity of HSC/MPPs. Glutathione (GSH) constitutes the most abundant pool of cellular antioxidants. Thus, GSH metabolism may play a critical role in hematological disease onset and progression.
A major limitation to studying GSH metabolism in HSC/MPPs has been the inability to measure quantitatively GSH concentrations in small numbers of HSC/MPPs. Current methods used to measure GSH levels not only rely on large numbers of cells, but also rely on the chemical/structural modification or enzymatic recycling of GSH and therefore are likely to measure only total glutathione content accurately.
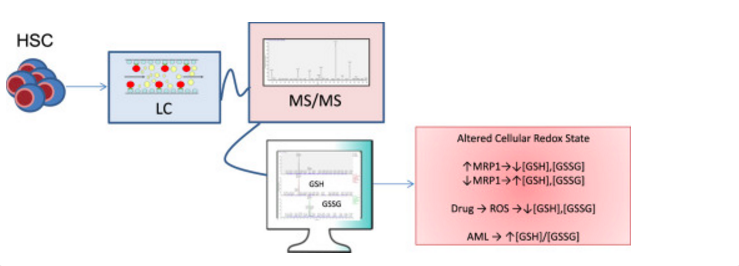
Here, we describe the validation of a sensitive method used for the direct and simultaneous quantitation of both oxidized and reduced GSH vialiquid chromatography followed by tandem mass spectrometry (LC-MS/MS) in HSC/MPPs isolated from bone marrow. The lower limit of quantitation (LLOQ) was determined to be 5.0 ng/mL for GSH and 1.0 ng/mL for GSSG with lower limits of detection at 0.5 ng/mL for both glutathione species. Standard addition analysis utilizing mouse bone marrow shows that this method is both sensitive and accurate with reproducible analyte recovery.
This method combines a simple extraction with a platform for the high-throughput analysis, allows for efficient determination of GSH/GSSG concentrations within the HSC/MPP populations in mouse, chemotherapeutic treatment conditions within cell culture, and human normal/leukemia patient samples. The data implicate the importance of the modulation of GSH/GSSG redox couple in stem cells related diseases.
— Published
Categories: Free Radical Biology and Medicine, Redox Biology, Research
If you’re an older adult, a 30-minute workout may not be as effective, even at the cellular level, as it was when you were younger. According to a new study, age may play a significant role in cell’s ability to respond to that activity.
The study, led by Dr. Tinna Traustadóttir of Northern Arizona University, was recently published in Free Radical Biology and Medicine, a journal of the Society for Redox Biology and Medicine (SFRBM).
In the study, a group of men age 18-30 were tested against a group of older men 55 years of age and older. Study participants were generally healthy, non-smokers, who were not taking antioxidant supplements in excess of a multivitamin, or any non-steroidal anti-inflammatory drugs for two weeks leading up to their study visit.

The two groups cycled for 30-minutes, with blood being drawn at six time points to test cell function and antioxidant response. For this study, the exercise intensity was set relative to the individual’s maximal aerobic capacity as determined by a maximal oxygen consumption test during screening. It is known that older adults have a reduced aerobic capacity compared to young, thus this allowed the group to maintain the same relative workloads between young and old test groups.
“Through this study we were able to determine that an individual’s antioxidant response to exercise becomes suppressed with age,” said Dr. Traustadóttir an Associate Professor at Northern Arizona University and SFRBM Member. “Exercise is effective and critical for people of all ages, but this study shows that older adults do not achieve the same beneficial cellular responses as younger adults from a single bout of moderate exercise.”
The findings indicate a single session of submaximal aerobic exercise is sufficient to activate a important group of antioxidant genes at the whole cell level in both young and older adults. However, nuclear import of Nrf2, the regulator for this group of antioxidant genes, is impaired with aging. Nuclear import is required for Nrf2 to access the antioxidant gene targets. Together these data demonstrate for the first time the weakening of Nrf2 activity in response to exercise in older adults.
Traustadóttir’s ongoing research aims to identify molecular processes responsible for age-related cellular changes. By better understanding the molecular signals promoting beneficial effects of exercise, definitive recommendations could be made for improving the body’s reaction to oxidative stress, which could lower the risk for many chronic diseases.
— Published
Categories: Free Radical Biology and Medicine, Research

Post by: Peter Vitiello, PhD
Last week, HBO’s satirical comedy newsman, John Oliver, addressed how scientific studies can be misconstrued by the media, transforming their original findings into entertainment solely suitable for morning show banter. In addressing how junk science becomes overhyped, the episode did a great job exposing data replication issues limiting research investments.
(Click Here to View HBO's John Oliver Video Clip. Warning: Language may be inappropriate for some.)
Reproducibility became an important topic of discussion in 2012 when Amgen reported that they were unable to reproduce findings in 47 of 53 “landmark cancer papers” published in journals with an impact factor greater than five. Even when considering failure rates of pre-clinical studies, this 94% irreproducibility rate was shocking and served as the motivation for new standards. The US National Institute of Neurological Disorders and Stroke called for data reporting standards in regard to animal randomization, blinded assessment, sample-size estimation, and data handling and biological sex and reagent authentication have also been added to this list. However, there was also a clear delineation between how this rigor should be applied exploratory studies (early-stage observational tests) and more robust hypothesis-testing experiments.
In light of such issues, direct requests to access raw data and protocol details of published studies have increased. However, editors of the New England Journal of Medicine, which coincidentally has the highest retraction rate of any journal in the world, recently refereed to such scientists as “research parasites”. Casey Greene at the University of Pennsylvania Perelman School of Medicine used this opportunity to create the Research Parasite Award to recognize outstanding contributions to the rigorous secondary analysis of data (nominations are due by October 14). More transparent approaches for handling confirmatory and replicative studies performed by such “parasites” are being developed. Although Amgen’s 47 irreproducible studies remain anonymous, they recently released data on three failed studies through a new “Preclinical Reproducibility and Robustness” channel created by Faculty of 1000. Many other publishers are following suit as Elsevier recently announced a new “Invited Reproducibility Paper” as a new article type appearing in the data science journal, Information Systems.
In summary, I’m very excited to see greater data sharing and transparency through guidelines set by both funding agencies and publishers along with opportunities and respect for parasitic confirmatory studies. I hope that scientists embrace such expectations and do not use them as sole justification to dismiss creativity and novelty during peer review. My only fear is knowing that my harshest critics watch John Oliver and I’ll be expected to respond to these cynics at our next family gathering.
— Published
Category: Redox Biology