By: Eugenio Barone and Marzia Perluigi
Read the full articles in Free Radical Biology and Medicine here: Barone E et al. Free Radic Biol Med, 111:262-269, 2017, Barone E et al. Free Radic Biol Med, 114:84-93, 2018
Accumulation of oxidative damage is a common feature of neurodegeneration that, together with mitochondrial dysfunction, point to the fact that reactive oxygen species are major contributors to loss of neuronal homeostasis and cell death. Among several targets of oxidative stress, free radical-mediated damage to proteins is particularly important in aging and age-related neurodegenerative diseases. In the majority of cases, oxidative stress mediated post-translational modifications cause non-reversible modifications of protein structure that consistently lead to impaired function.
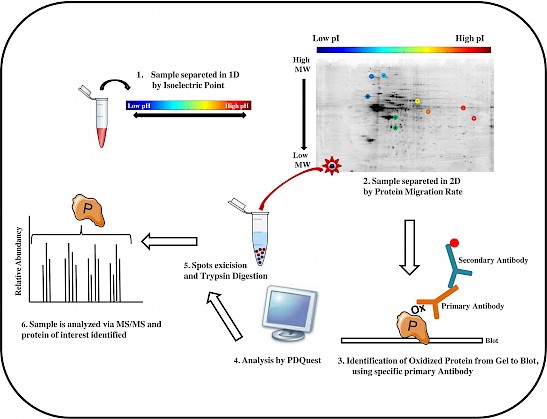
Figure: The workflow of a redox proteomics analysis is showed in six principal steps.
Redox proteomics methods are powerful tools to unravel the complexity of neurodegeneration, by identifying brain proteins with oxidative post-translational modifications that are detrimental for protein function and pathogenesis (Butterfield DA et al. Biochem J, 463:177-89, 2014). Our published studies show evidence of impaired pathways linked to oxidative stress possibly involved in the neurodegenerative process leading to the development of Alzheimer-like dementia (Di Domenico F et al. Antioxid Redox Signal, 26:364-387, 2017). Recently, we also focused on dysregulated pathways underlying neurodegeneration in aging adults with Down syndrome (DS) (Barone E et al. Free Radic Biol Med, 111:262-269, 2017). Interestingly, DS individuals by the age of 40ys are at increased risk to develop Alzheimer disease (AD) like dementia.
Results obtained by the analysis of human specimens and studies from mouse and cellular models of the disease evince a molecular link between protein oxidation/aggregation, the integrity of protein quality control system [proteasome, unfolded protein response (UPR) and autophagy], dysfunction of energy metabolism and neurodegeneration (Di Domenico F et al. Antioxid Redox Signal, 26:364-387, 2017). Many common pathological hallmarks exist between DS and AD including deposition of b-amyloid plaques, Tau-based neurofibrillary tangles, increased oxidative damage, and impaired mitochondrial function, among others. Intriguingly, we propose that all these processes seem to be joined by a “leitmotif” – oxidative stress - since they are all the cause and/or the consequence of increased free radical burden. If low amounts of reactive oxygen species (ROS) can activate the protective cellular apparatus such as the antioxidant and heat shock responses, cell cycle regulation, DNA repair, UPR and autophagy, then chronic exposure to ROS causes irreversible damage to all intracellular macromolecules (Barone E et al. Free Radic Biol Med, 111:262-269, 2017). Among these, protein oxidation impairs multiple cellular functions by a largely irreversible process that results in altered, mostly reduced, protein activity. It is likely that stressed neurons have the challenge of increasing loads of oxidatively damaged proteins, which overwhelm the ability of the proteostasis network. This, in turn, promotes further accumulation of damaged proteins, increasingly prone to aggregation, ultimately resulting in neuronal death. Alteration of protein homeostasis coupled with increasing demand for protein degradation, and reduced ATP production may produce a vicious cycle that may accelerate the neurodegenerative process (Barone E et al. Free Radic Biol Med, 111:262-269, 2017, Barone E et al. Free Radic Biol Med, 114:84-93, 2018).Therapeutic strategies aimed at preventing/reducing multiple components of processes leading to accumulation of oxidative damage will be critical in future studies.
Laboratory of Redox Biochemistry in Neuroscience, Department of Biochemistry, Sapienza University of Rome, Email: Eugenio.barone@uniroma1.it
— Published
Category: Redox Biology